CNIPA Order No. 391: Revising the Chemical Invention Chapter in Patent Examination Guidelines
Beginning from May, 2020, China National IP Administration (“CNIPA”) launched a new program aimed to comprehensively revise the Patent Examination Guidelines. Segmented into different series, the new program released its first series of revisions, which related to Chapter 10 of the Part 2 of the Guidelines dedicated to chemical inventions. After reviewing the public input, CNIPA promulgated the first series of revisions as its Order No. 391 which has come into force on January 15, 2021 (hereinafter referred to as “Revisions”). Here are the main paragraphs touched upon in the first series.
Post-filing data supplement for chemical inventions has been allowed since 2016. Admissible data are those derivable from the as-filed disclosure by a person having ordinary skill in the art, in order to support the provable technical effects available in the disclosure. But it has been criticized by some patent practitioners that CNIPA was strict to determine what constitutes the admissibility of post-filing data. The Revisions now make it clear and emphasize that if the post-filing data are filed for the purpose of sufficient disclosure/enablement (§22(3)) and inventiveness (§26(3)), those will be accepted. For instance, an application claims a compound X, along with very detailed descriptions including examples to prepare the compound X, the beneficial effects of hypotension, and the method to measure hypotensive activity. But there was no experimental data in the patent specification. Experimental data filed after filing should be considered for sake of supporting the requirement of sufficient disclosure.
The Revisions rephrased the guidance for novelty. A compound is not novel if its structural information such as name, molecular formula, etc. has been stated in a prior art reference detailed in a level that a person having ordinary skill in the art considers the compound being made public. It cannot be forthrightly deemed loss of novelty when only, as the Guidelines used to state, a reference “mentions” the compound. Besides, the source of prior art can be combined from different pages in one single document. When the compound’s structural information in one cited reference is not enough to determine identity of compounds but other information including physical and chemical properties, synthetic methods, and experiment data are available to corroborate such identical compounds, a person having ordinary skill in the art may presume that the two compounds are essentially the same so that the cited prior art reference anticipates. Yet, the applicant can argue them being different by submitting more other evidence.
For determining inventive step, the Revisions highlighted the three-step test (or similarly the problem-solution approach), unexpected results, and technical suggestions, as follows.
(1) one shall firstly identify the structural difference between the prior art and the claimed compound as well as the technical problem to be solved by the structural modification’s usage and/or effect, so as to determine whether the prior art reveals any technical suggestions. Particularly when the person having ordinary skill in the art can derive the claimed compound by reasoning, analysis, or limited experiment based on the cited prior art reference, said prior art introduces technical suggestions.
(2) The claimed compound’s structural modification from the closest known compound can bring about different usage or can be an improvement in effect. When the different usage or improvement in effect is unexpected, the claimed compound should be non-obvious in light of prior art and hence be inventive.
(3) If the claimed invention’s technical effect is known and inevitable, the claimed compound is not inventive.
For one instance,
Prior art (Compound Va) is 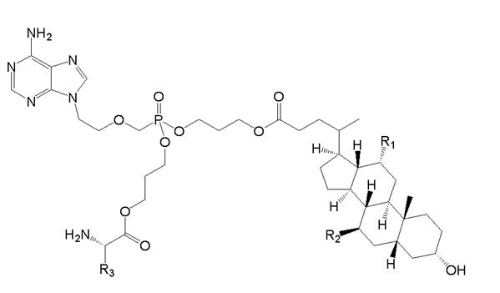
, wherein R1=OH, R2=H, and R3=CH2CH(CH3)2.
Claimed invention (Compound Vb) is 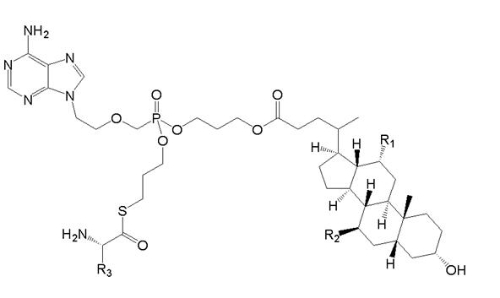 , wherein R1 and R2 can be H or OH, R3 is a C1-6 alkyl group, whereas Vb includes a specific compound Vb1 where R1=OH, R =H, and R3 = CHCH3CH2CH3. Compound Vb1 has an anti-hepatitis B virus activity superior than compound Va does. , wherein R1 and R2 can be H or OH, R3 is a C1-6 alkyl group, whereas Vb includes a specific compound Vb1 where R1=OH, R =H, and R3 = CHCH3CH2CH3. Compound Vb1 has an anti-hepatitis B virus activity superior than compound Va does.
In view of compound Va versus compound Vb, the only difference is that the linking atom between the amino residue and the phosporyl group – sulphur (-S-) for compound Vb and oxygen (-O-) for Va. Since the chemical properties of sulphur and oxygen are similar, a person skilled in the art has a motivation to undergo a substitution of atoms from oxygen to sulphur in order to attain compound Vb. Hence compound Vb does not have inventive step. In the contrary, compound Vb1 is different from compound Va by not only the linking atom but also the R3 group. Moreover, compound Vb1 possess a significantly superior activity against hepatitis B virus. In the prior art there is no such a technical suggestion to improve anti-hepatitis B virus activities by the indicated modifications in chemical structure. Hence compound Vb1 has inventive step.
The section for biotechnological inventions is another focus in the Revisions. To draft a patent claim, a monoclonal antibody can now be defined by its structural limitations, in addition to defining by only the hybridoma which generates the claimed monoclonal antibody. This change accommodates well the development in amino acid sequencing technology where the structural data of monoclonal antibodies are becoming more easily identifiable.
The three-step test is again emphasized in the sections for biotechnology inventions. That is, the examiner is advised to determine the difference between the claimed invention and the closest prior art, and then to identify the technical problem to be solved by the technical effect of the claimed invention. Next, the examiner shall ascertain whether the prior art introduces a technical suggestion. Finally, based on a found technical suggestion, the examiner shall resolve whether the claimed invention is obvious in light of the prior art. This approach is surely applicable to various kinds of biotech/genetic subject matters such as genes, peptides, proteins, recombinant vectors, transformed DNAs, fusions cells, monoclonal antibodies.
|